
Protons are present within the atomic nucleus and are positively charged.
He also stated that there was a neutral particle which later on, his student, James Chadwick, confirmed in the year 1932. In 1920, he was the first one to propose the name proton for positive charged atomic particles. Protons and neutrons have almost the same mass.Įrnest Rutherford first discovered the nucleus in 1911, as stated by the American Institute Of Physics. Protons and neutrons are a lot heavier than electrons and reside within the atom's nucleus. Quarks then came together to form protons and neutrons. As the earth cooled down, it led to the formation of quarks and electrons. DEFINING NUCLEUSĪtoms were formed after the Big Bang theory, which happened 13.7 billion years ago.
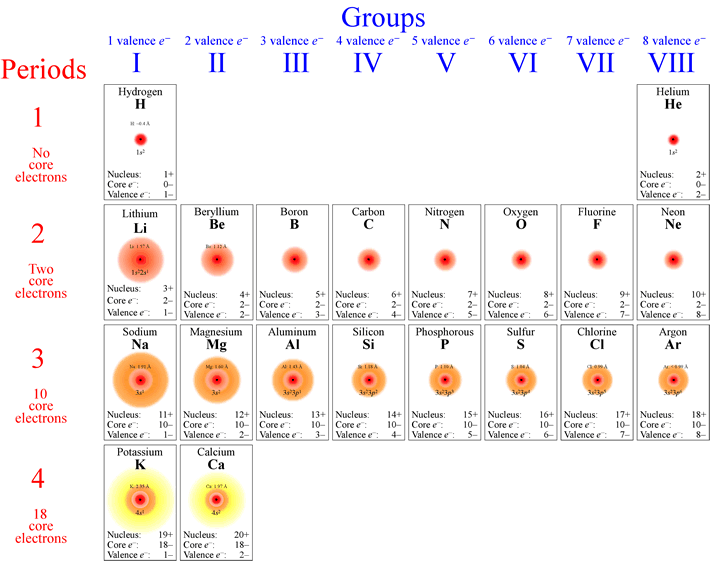
However, you must have heard of electrons, protons, neutrons? But what are they? And, how are they related to atoms? Atoms can be divided, and they can be divided into subatomic particles called protons, electrons, and neutrons which can again be divided into quarks. Where does the word atom come from? Atom comes from the classic Greek word for indivisible because it was thought then that atoms are the smallest universal thing and they cannot be further divided. Have you ever wondered what your body is made up of? What about the ball you play soccer with? Ever wondered what plants are made of? You might have heard the word 'atom,' but do you know what it is? Atoms are considered the basic units of matter and everything on the earth is made up of atoms except for energy.


 0 kommentar(er)
0 kommentar(er)
